A Particle That Moves Around the Nucleus Is a N
A proton is positively charged and is located in the centre or nucleus Of the atom. Two protons and and two neutrons vibrating in some fashion inside the nucleus.
What Is Revolving Around The Nucleus Of An Atom Quora
A particle with a charge of 5 106 C and a mass of 20 g moves uniformly with a speed of 7 ms in a circular orbit around a.

. Electron around nucleus is described by complex wave function with both real and imaginary partsThe electron is no longer described as moving around the nucleus but is found with a certain probability around the nucleus as given by the Schrodingers equation. Electrons are electronegatively attracted to the nucleus and play a big part of the elements properties and bonding behavior. Nuclear Stability More than 1000 nuclides have been identified but not all are stable.
1 Answer Al E. The black circles represent the stable nuclides. 25 Questions Show answers.
A negatively charged particle that occupies the space in an atom outside the nucleus is called an. They are infinitesimally small relative to the nucleus. 4 Which particles move around.
It refers to the energy level of the oscillating spherical electric field surrounding and bound to an atomic nucleus. A stable nucleus maintains its constitution all the time. Explanationhope it help and plz mark the brainliest.
The nucleus is not a classical world. Chemistry Matter Basic Atomic Structure. The nucleus contains two types of subatomic particles protons and neutrons.
Here probability is a measure of chance of finding an electron over a region. The subatomic particle that moves around in the. A third type of subatomic particle electrons move around the nucleus.
Secondly what charge does the nucleus have. The negatively charged subatomic particle that moves around the nucleus is the electron. Electrons exist in a cloud around the.
The protons have a positive electrical charge and the neutrons have no electrical charge. The __________ contains the protons and neutrons of an atom and is most of the mass of an atom. Each particle that moves around this center is called an 4_____.
The subatomic particle that moves around in the. 1862 students attemted this question. These two particles form a dense center called the 3_____.
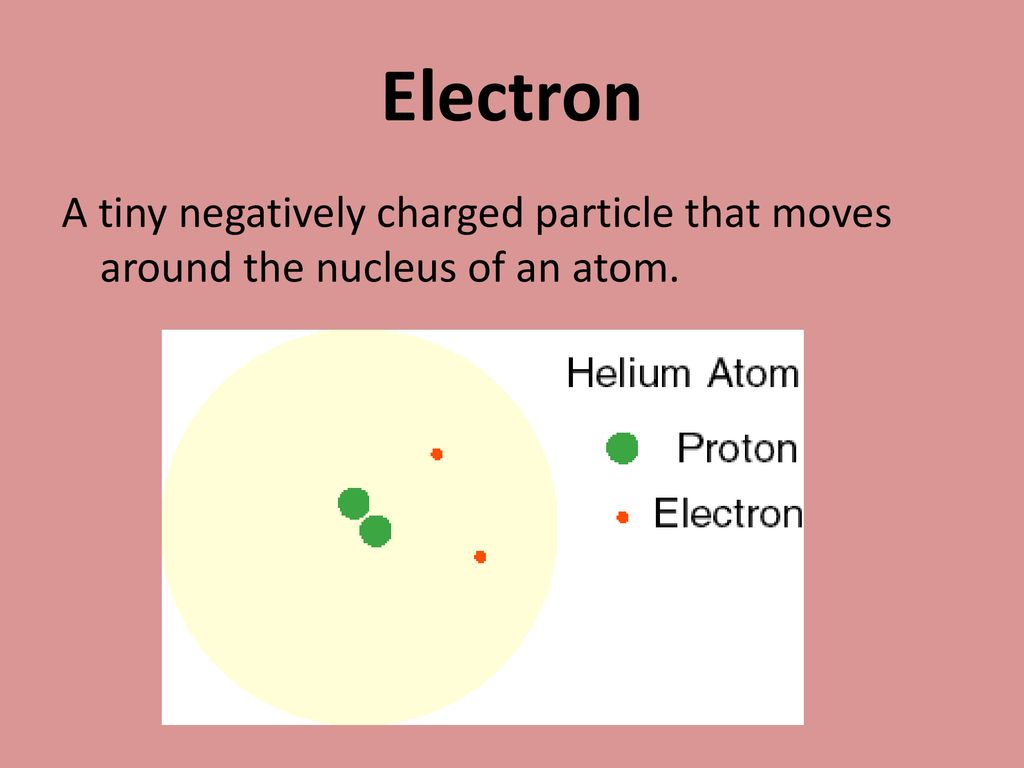
The protons have a positive electrical charge and the neutrons have no electrical charge. A particle that moves around the nucleus is a n. Electrons are negatively charged and are located in rings or orbits spinning around the nucleus.
A negatively charged subatomic particle that moves around the nucleus is a n. What is a particle that moves around the outside of the nucleus. What carries no charge.
A particle that moves around the nucleus is an. A particle that moves around the nucleus is a n. The electrons have a negative electrical charge.
The nucleus of an atom is charged. 2 Which two particles are found in the nucleus of an atom A neutrons and electrons B protons and electrons c protons and neutrons d neutrons and atoms. Niels Bohr worked with Ernest Rutherford on atomic structure.
Is used to accelerate protons in the study of subatomic particles. A particle that moves around the nucleus is a n. In the middle of every atom is the nucleus.
Write the letters of the correct answers on the lines at left. The central region of an atom where its neutrons and protons are is its. 3 Which subatomic particles are found in the nucleus of an atom of beryllium and what is the net charge of the nucleus.
Protons and Neutrons are part of the nucleus. The proton along with the neutron comprises the nucleus. An unstable nucleus emits some kind of particle and changes its constitution.
Neutrons have a negative charge. The particles that orbit around the nucleus is an electron. It is found in the empty space of an atom according to the experiment conducted by Lord Rutherford and his two collaborators Geiger and Marsden in 1909 as they bombarded the nucleus of tin gold foil with al.
Particles in an atom that are neutral and have no charge are. 1 What two particles are found in the nucleus of an atom. The nucleus contains two types of subatomic particles protons and neutrons.
Electrons are the particles that revolve around the nucleus in orbits called shells. A nucleus is made. A third type of subatomic particle electrons move around the nucleus.
The electrons have a negative electrical charge. Electrons are not a part of the nucleus and they revolve around it in fixed paths known as orbits. There are many orbital levels in which electrons can exist.
As soon as you classically separate the two protons you are going to get an electric dipole moment no matter where you place them and no matter how you arrange them to vibrate. Up to 24 cash back A positively charged particle in an atom is an 1_____ and a particle with no charge is an 2_____. Figure 461 shows a plot of neutron number N versus proton number Z for the nuclides observed.
20 Questions Show answers. Hence a third Type of subatomic particle electron move around the nucleus. He discovered that electrons moved in specific orbits around a positive nucleus.
The positive particles of an atom are.

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets
Nucleus The Central Core Of An Atom The Nucleus Contains Two Types Of Particles Positively Charged Protons And Usually Neutral Neutrons Ppt Download

No comments for "A Particle That Moves Around the Nucleus Is a N"
Post a Comment